Abstract
Background: Until the advent of targeted agents, the primary treatment for patients with relapsed or refractory (R/R) chronic lymphocytic leukemia (CLL) with 17p deletion (del(17p)) was allogeneic hematopoietic stem cell transplantation (HSCT). Venetoclax is a targeted therapy that has been shown to effect significant progression free (PFS) and overall survival (OS) with an acceptable safety profile and the convenience of oral administration. HSCT is potentially curative but is associated with potential transplant-associated morbidity and mortality (incl. graft-versus-host disease) and relapse. Both treatments are expensive, HSCT largely up-front and venetoclax long term, with relative differences in cost of treatment and supportive care management. Considering these different efficacy, safety, and procedural profiles, we evaluated the cost-effectiveness and cost-utility of venetoclax versus HSCT in R/R CLL del(17p) from a US payer perspective.
Methods: A life time horizon Markov model was constructed specifying 3 health states: PFS with embedded sub-states specifying whether patient is on or off therapy, progression, and death. Data sources to simulate the R/R CLL del(17p) patients included Stilgenbauer et al (Lancet Oncol 2016) for venetoclax and Schetelig et al (J Clin Oncol 2008) for HSCT. Kaplan-Meier PFS and OS curves were digitized, PFS and OS data extracted, and Weibull distributions fitted to these data for both venetoclax and HSCT. The wholesale acquisition cost of venetoclax was sourced from RedBook. Costs of the HSCT procedure, pre-conditioning treatment, and post-procedural side effects were estimated from published prediction equations and national private claims database. EQ-5D utility values were sourced from published literature. Disutility values for side effects were assumed same for both interventions. A discount rate of 3% was applied when survival exceeded one year. Following extrapolation to a life time horizon, the life years (LY) and quality adjusted LY (QALY) for each treatment were estimated, and the incremental LY and QALY gained with venetoclax over HSCT calculated. From the respective total treatment costs, cost differentials and the incremental cost effectiveness (ICER) and cost utility ratios (ICUR) were determined. Base case analyses were validated by probabilistic sensitivity analyses (PSA) of 2000 iterations.
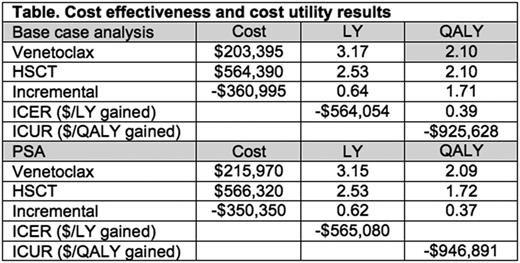
Results: Venetoclax prevailed in PFS over HSCT until ~190 weeks (3.7 years) when curves crossed and HSCT prevailed. Venetoclax prevailed in OS over HSCT over the full life time horizon. As shown in the Table, in the life time Markov model, compared to HSCT, venetoclax showed cost savings of -$360,995 (PSA: -$350,350) and incremental gains of 0.64 (PSA: 0.62) LY and 0.39 (PSA: 0.37) QALY. This yielded an ICER of savings of -$564,054 (PSA: -$565,080) per LY gained and an ICUR of savings of -$925,628 (PSA: -$946,891) per QALY gained.
Conclusion: Against a life time horizon, venetoclax prevails in OS and for ~3.7 years in PFS over HSCT in the management of patients with R/R CLL del(17p). Yielding a cost saving by ~$565,000 if LYs gained are concerned but by ~$945,000 in the case of QALYs gained, it has the life time benefit of being clinically superior in combined OS and safety at markedly lower cost. While surviving HSCT-treated patients had improved outcomes with a slightly higher likelihood of subsequent PFS than venetoclax-treated patients, this marginal benefit is dominated by the significantly higher probability of OS for patients with venetoclax therapy. Venetoclax provides significantly greater life time clinicoeconomic value in the treatment of patients with R/R CLL del(17p) versus HSCT.
Persky: Genentech: Consultancy; MorphoSys: Other: Independent Data Monitoring Committee member ; Verastem: Consultancy; Spectrum Pharmaceuticals: Research Funding. Abraham: Janssen Oncology (Johnson & Johnson, LLC): Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.